Science makes your fireworks | Weather Blog
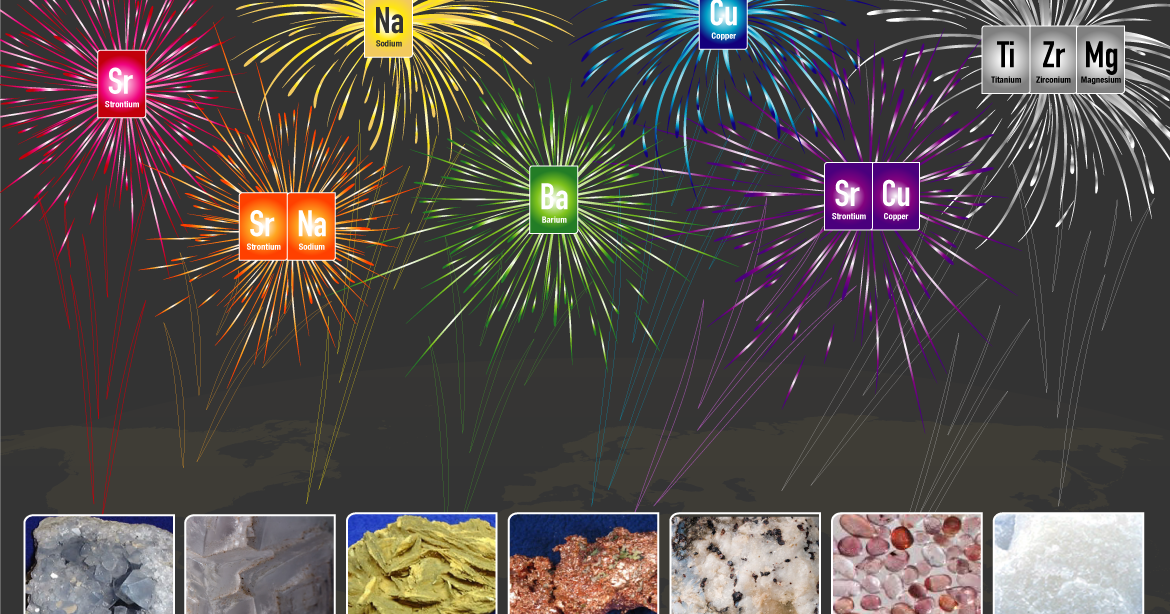
As you watch fireworks light up the sky this holiday weekend, think about the science involved in the spectacle. The United States Geological Survey shares which minerals create the different colors you see and what else these minerals are used for:
Red: Sr – Strontium
Orange: Sr – Strontium, Na – Sodium
Yellow: Na-Sodium
Green: Ba – Barium
Blue: Cu – Copper
Violet: Sr – Strontium, Cu – Copper
Gray and White: Ti – Titanium, Zr – Zirconium, Mg – Magnesium
The gold sparks are produced by iron filings and small pieces of charcoal. Bright flashes and loud bangs come from the aluminum powder.
STRONTIUM*: In addition to its use in making fireworks, strontium is used in signage, oil and gas production, and ceramic magnets.
SODIUM: In addition to turning our fireworks yellow, sodium is used to make polyvinyl chloride (PVC) plastic made from chlorine and pulp chemicals made from caustic soda.
BARIUM CHLORIDE*: In addition to making fireworks green, barium is also used in medicine and in oil and gas production.
COPPER: In addition to making fireworks blue and purple, copper is one of the oldest metals used by humans, and today is primarily used in electronics and power generation.
TITANIUM*: Along with zirconium and magnesium to make gray and white fireworks, titanium is widely used as a white pigment and in metal alloys.
ZIRCONIUM*: Along with titanium and magnesium to make gray and white fireworks, zirconium is used in high temperature ceramics industry.
MAGNESIUM*: Along with Titanium and Zirconium to make gray and white fireworks, Magnesium is used in furnace linings for the manufacture of steel and ceramics.
* Critical mineral product
When you’re the smartest person at the fireworks, thank the USGS. And send us a photo on social media or via the WDRB weather app!